Le Châtelier Was Trying to Show Us Something Bigger—Did You Miss It?
Psst... Le Châtelier might be my favorite chemist now.
Most people learn Le Chatelier’s Principle by memorizing a table of rules:
"Increase reactants? Shift right."
"Increase pressure? Shift to fewer moles of gas."
"Increase temperature? Depends on exo/endothermic."
But that’s completely missing the point. (and honestly, quite exhausting)
Le Châtelier wasn’t sitting around to make you memorize a list of rules. Nah.
He wanted to show us how systems naturally respond to stress.
If you don’t see that, you’re missing everything.
I made this visual breakdown that walks through equilibrium step by step.
This isn’t just another reference sheet—it’s a way of thinking that lets you discover it for yourself.
We’re going to tackle (or retackle) this concept in my favorite way.
Observe. Find a pattern. Use it over and over — until it breaks.
Then make a better pattern.
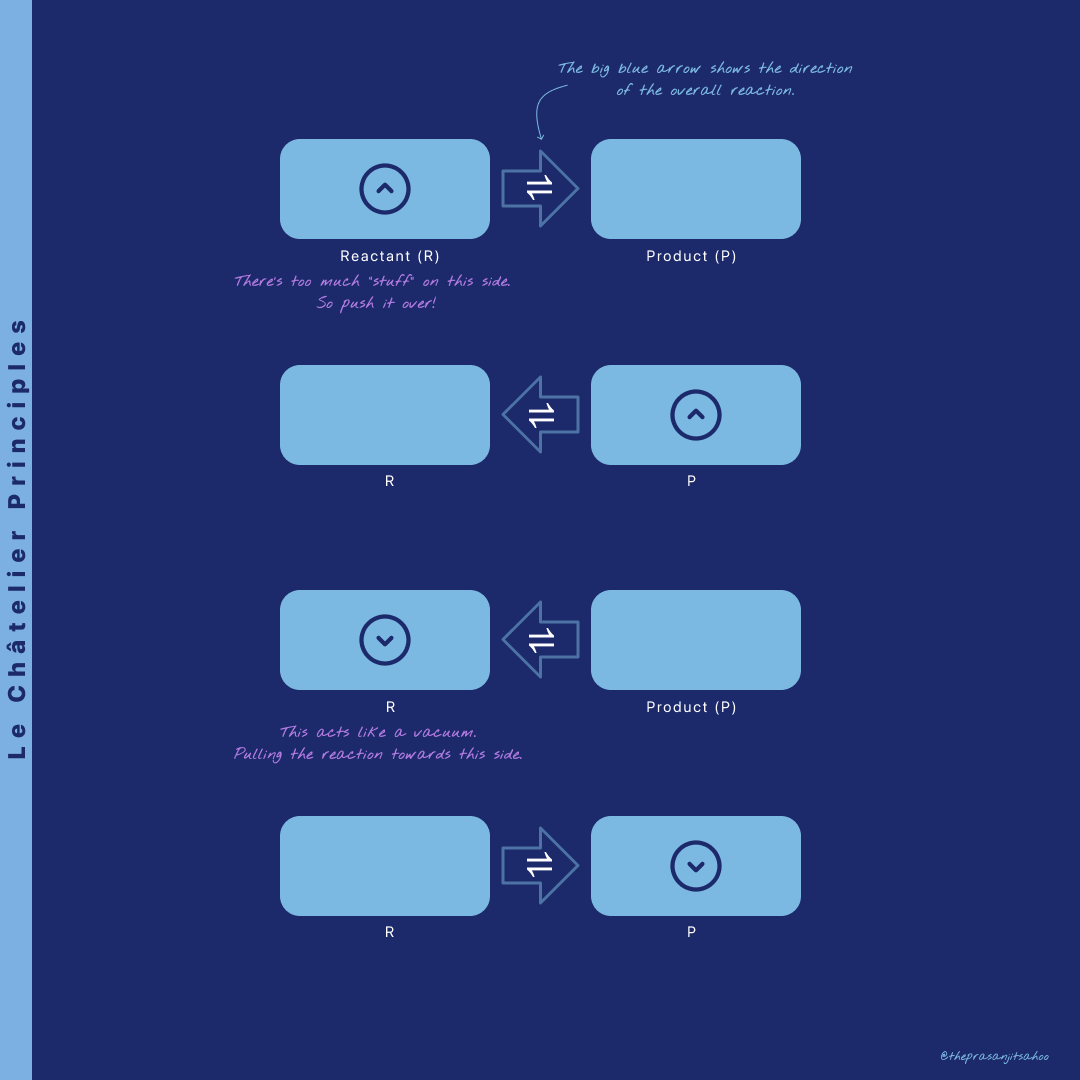
Stage 1
Ready? Here’s Stage 1: Le Châtelier Principles for Concentration. Just observe what’s going on, and then try finding a pattern. Treat it like a game. In purple, I’ve wrote down my own thoughts.
Okay, so that was Stage 1. I’m pretty sure you’re noticing this “too much stuff” and “vacuum” idea. Let’s see if this holds and stays true for the other stages.
Stage 2
Here is Stage 2. Textbooks start overcomplicating it here — trying to classify “endothermic/exothermic” reactions immediately. You don’t need to do that.
Again, just observe and find a patterns.
Okay, that was Stage 2. Seems like our idea (the “too much stuff” and “vacuum”) holds true. Maybe we’re just lucky.
I will add that a lot of textbooks/professors build in the concept of exothermic and endothermic reaction when talking about heat.
We already know that endothermic reactions give off heat, so that would be heat in the reactants. Hence, that’s the first equation.
Vice versa, we already know that exothermic reactions give off heat, so that would be heat in the products. Hence, that’s the second equation.
Ultimately, create heat like “extra/less concentration of the reactant or product” then classify the reaction.
Stage 3
Okay, now the final stage: Stage 3. Observe and find a patterns.
Okay, so this time our pattern did not prevail (but it did for the past two situations!). It seems like pressure works a bit different. When we get pressure, we want to consider volume at the same time (∵ PV=nRT). That way we know which way the direction the equilibrium shifts.
Side note: If the number of gas moles is the same on both sides, changing the pressure or volume has no effect on equilibrium.
Congratulations You Understand Dynamic Equilibrium!
Concentration → More pushes over, Less vacuums in
Temperature → More pushes over, Less vacuums in → Determine exothermic/endothermic
Pressure → Volume!
Here’s what you understand: Dynamic Equilibrium is a dance that works towards balance.
Because you understand this, you’ll never need to brute-force a table again (or more Anki cards, tho it’ll help this stick).
In fact, you did great! You saw a pattern, you applied the pattern, you refined the pattern.
Vidi, Applicavi, Refinavi.
Here’s the whole thing altogether now (but do you really need it now?)
Did this give you an "A-ha!" moment? I hope you have a deeper understanding of Le Châtelier Principles now! I’ve thrown in 3 MCAT Style questions below — write you answers in the comments! Let's get this 528!
3 MCAT Style Questions
A student adds extra H₂ gas to the following equilibrium reaction at constant temperature:
N2(g)+3H2(g)⇌2NH3(g)+heat
According to Le Chatelier’s Principle, what will happen?
A) The reaction will shift left, increasing N2N2 concentration.
B) The reaction will shift right, increasing NH3NH3 concentration.
C) The reaction will shift left, decreasing temperature.
D) The reaction will not shift, since equilibrium is unaffected by concentration changes.
The following reaction is exothermic:
2SO2(g)+O2(g)⇌2SO3(g)+heat
Which of the following changes will shift the equilibrium toward the reactants?
A) Increasing temperature
B) Decreasing volume
C) Adding SO₂ gas
D) Removing heat
A closed system contains the following reaction at equilibrium:
CaCO3(s)⇌CaO(s)+CO2(g)
What happens if the volume of the container is suddenly decreased?
A) The reaction shifts left, increasing CaCO₃.
B) The reaction shifts right, increasing CO₂.
C) The reaction does not shift because solids are present.
D) The reaction does not shift because volume changes only affect liquids.